Source: Ministry of Public Security, State Taxation Administration of The People’s Republic of China, etc.
Cartography: Cai Huawei
"With the effective control of the epidemic and the effectiveness of various policies and measures, automobile consumption will continue to stabilize and recover, and automobile consumption is expected to achieve rapid growth in the second half of the year." On July 7th, at the the State Council policy briefing, Vice Minister of Commerce Sheng Qiuping introduced the work related to increasing automobile consumption steadily.
Break the institutional obstacles that restrict the development of automobile circulation
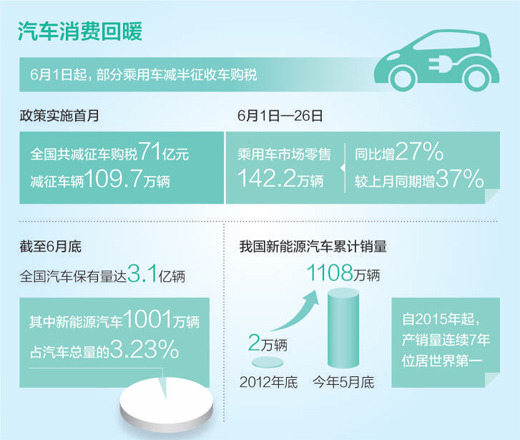
In 2021, the national retail sales of automobile products reached 4.4 trillion yuan, accounting for 9.9% of the total retail sales of consumer goods, which is an important support of the consumer market. Since April this year, due to multiple factors, the downward pressure on the automobile consumption market has increased. The Ministry of Commerce, in conjunction with relevant departments, studied and promulgated "Several Measures on invigorating automobile circulation and expanding automobile consumption" (hereinafter referred to as "Several Measures").
Highlight the construction of a unified national automobile market. According to the "Several Measures", we will break the local protection of the new energy vehicle market, support new energy vehicles to go to the countryside, cancel the restrictions on the movement of used cars nationwide, and optimize and improve the transfer registration of used cars.
Highlight the optimization of the automobile use environment. Accelerate the improvement of automobile use environment and promote the transformation of automobile from purchase management to use management. For example, in solving the urban parking problem, it is required to actively expand new parking facilities in combination with urban renewal actions; Make rational use of civil air defense projects, underground spaces of parks and green spaces, and tap the potential to build more parking facilities.
Highlight the promotion of green and low-carbon cycle development. Support the purchase and use of new energy vehicles, and further increase the sales proportion of new energy vehicles; Support the improvement of the recycling system of scrapped motor vehicles, promote the recycling of resources, and guide the automobile market to accelerate the green transformation and upgrading from the front and rear ends of automobile sales and scrapping updates.
Highlight the promotion of consumption in all areas of the automobile chain. Focus on the whole process of new car sales, second-hand car trading, scrap car recycling, parallel import of cars, automobile cultural tourism consumption, automobile financial services, etc., and strive to "pull increments, revitalize stocks, smooth circulation, and drive linkages" to fully release the potential of automobile consumption.
"In general, these measures are highly targeted and operational, focusing on breaking down some institutional and institutional obstacles that have long restricted the development of automobile circulation, consolidating the trend of automobile consumption stabilization, and promoting the transformation and upgrading of the automobile market." Sheng Qiuping said.
The policy of restricting the removal of used cars will be completely abolished from next month.
Used cars are an important link in the whole life cycle of automobiles. Sheng Qiuping said that invigorating the circulation of used cars can revitalize the stock of cars and boost the consumption of new cars, which plays a very important role in promoting the consumption of cars.
In 2021, China’s second-hand car trading volume reached 17.59 million, less than 6% of the total car ownership, far below the proportion of the international mature car market. The second-hand car market is seriously hindered by the problems of cross-regional restrictions on moving, unclear commodity attributes and limited distribution business.
"The Ministry of Commerce has put forward a series of measures to comprehensively develop the circulation of used cars, hoping to completely get through the difficulties of blocking points." Sheng Qiuping said.
Completely cancel the policy of restricting relocation. According to the "Several Measures", since August 1, 2022, the restrictions on the movement of small non-operating used cars that meet the national five emission standards will be lifted nationwide, so as to promote the free circulation of used cars, facilitate enterprises to operate across regions and facilitate people to buy and sell used cars in different places.
Optimize transaction registration management. The "Several Measures" clarify that the second-hand cars distributed by enterprises should be accounted for as "inventory goods" like new cars, and a separate endorsement should be made at the time of motor vehicle transfer registration, and a temporary driving vehicle number plate should be issued, which greatly optimizes the transaction registration process; For cities where automobile purchases are restricted, it is clear that the second-hand cars purchased by automobile sales enterprises and used for sales do not occupy the number plate index, further reducing the operating costs of enterprises and facilitating the circulation of second-hand car transactions.
Support the development of distribution business. China’s used car market is dominated by the "broker+individual" model, and the problem of "small and weak" in the industry is prominent. To this end, the "Several Measures" require the abolition of unreasonable restrictions on the development of second-hand car distribution, and it is clear that enterprises with registered residences and business premises outside the second-hand car trading market can carry out second-hand car sales business.
In terms of promoting automobile renewal consumption, Xu Xingfeng, director of the Department of Market Operation and Consumption Promotion of the Ministry of Commerce, introduced: First, encourage old vehicles to be eliminated and updated, encourage all localities to comprehensively use economic and technological means to promote the withdrawal of old vehicles, and carry out automobile trade-in in in areas where conditions permit. The second is to improve the recycling system of scrapped motor vehicles and support advanced enterprises to enter this system, thus promoting the standardized and healthy development of the recycling and dismantling industry of scrapped motor vehicles; Help market players reduce their burdens and extend the transition period for the qualification re-certification of scrapped motor vehicle recycling enterprises; Increase the support for the construction projects of scrapped motor vehicles recycling and dismantling enterprises, and make it clear that the land for enterprise construction projects is industrial land in principle.
In the first five months, 8940 charging piles were added in the renovation of old urban communities.
Guo Shougang, head of the Equipment Industry Division I of the Ministry of Industry and Information Technology, said that supporting the purchase and use of new energy vehicles is an effective way to stabilize and expand automobile consumption and ensure the smooth operation of the industry. In the next step, the promotion scale of new energy vehicles will be further expanded.
First, continuously improve the quality of product supply. We will continue to improve the safety technical standards for new energy vehicles, and improve the performance levels of thermal runaway alarm, safety protection and low temperature adaptation of power batteries, so that consumers can buy and use with confidence. Promote the integration of electrification and intelligent networking technology, develop more service functions suitable for consumers, and continuously improve the driving experience.
The second is to actively create a good use environment. Strengthen the monitoring and judgment of industry operation, do a good job in ensuring the supply and price stability of automobile chips and upstream raw materials, and fully ensure the smooth and stable supply chain of the industrial chain. Encourage qualified places to introduce policies to promote consumption with gold content. We will promptly study and clarify the support policies such as the continuation of tax incentives for new energy vehicles, optimize the "double points" management method, and do a good job in effectively connecting with subsidies.
Zhang Yan, head of the Department of Building Energy Conservation and Technology of the Ministry of Housing and Urban-Rural Development, said that in recent years, the Ministry of Housing and Urban-Rural Development has continued to promote the construction of charging facilities in residential communities and strongly supported the consumption of new energy vehicles.
Clear standards. A series of standards and specifications, such as "Standards for Planning and Design of Urban Residential Areas", were revised and issued, which clearly stipulated that "100% charging facilities should be built or installation conditions should be reserved for parking spaces in new residential buildings", and standards for planning and site selection and power supply system of charging facilities were formulated.
Make plans. Since 2021, 59 sample cities have been selected to carry out urban physical examination, and the coverage rate of community low-carbon energy facilities such as charging facilities has been listed as the content of urban physical examination, guiding sample cities to find shortcomings in the construction of charging facilities, and making overall plans to determine the construction goals, plans and projects of charging facilities.
Classified propulsion. For existing residential areas, all localities will be instructed to renovate or build charging facilities for electric vehicles in and around the residential areas, as part of the improvement of old urban residential areas. In the first five months of this year, 8940 charging piles were added in the renovation of old urban communities. For new residential areas, guide all localities to strictly implement the provisions of 100% charging facilities or reserved construction and installation conditions, and cooperate with relevant departments to seriously investigate and deal with the behavior of real estate development enterprises that fail to build charging facilities in accordance with regulations.
People’s Daily (July 8, 2022, 02 edition)















![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1JyAd7yGAAAAAAAAAAA656.640x366.jpg)
![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1JyATZnxAAAAAAAAAAA227.640x427.jpg)
![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1JyAeLxGAAAAAAAAAAA149.640x427.jpg)
![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1J2AGYpOAAAAAAAAAAA799.640x355.jpg)
![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1J2Ab71vAAAAAAAAAAA210.640x403.jpg)
![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1J2AN6kNAAAAAAAAAAA816.640x428.jpg)
![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1J2AGsUQAAAAAAAAAAA338.640x427.jpg)
![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1J2AWV2RAAAAAAAAAAA366.640x372.jpg)
![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1J2AUVyPAAAAAAAAAAA725.640x366.jpg)
![Car Channel [Feed] [News] "China Steel Cannon" PRO is coming, the price is 79,800 - 119,800 yuan, Geely Colorful PRO is officially listed](https://p2.cri.cn/M00/2D/18/wKgACl6W1J6ADkr5AAAAAAAAAAA483.640x427.jpg)