Established pharmaceutical companies learn from online celebrity brand. Have you ever bought such branded products?
Original Bajiuling Wu Xiaobo Channel
It may be difficult for pharmaceutical companies to embark on the road of OEM, but it is not a long-term solution after all.
Text/Ba Jiuling (WeChat WeChat official account: Wu Xiaobo Channel)
01
These things you bought online are probably branded products.
Have you bought scar cream, hair tonic, warm palace stickers, diet pills and other health care products online? If these products come from well-known pharmaceutical companies such as Renhe, Tongrentang and Xiuzheng, will you have more trust, even if its price is higher than similar products, will you pay for them?
But in fact, the products you bought are probably not from the pharmaceutical companies’ own factories, but are OEM products.
For example, Tmall’s "Medicine Capital Renhe Flagship Store" has a monthly sales of 10,000+and more than 7,000 comments. According to the product details page, this product is from Renhe Pharmaceutical, but it does not appear in the product list of Renhe Pharmaceutical official website.
According to the approval number of this product "G20180064", minibus found the registration information of this product in official website of the Food and Drug Administration, which showed that the manufacturer was "Guangzhou Aifuyi Biotechnology Co., Ltd.", and the last remark information also stated that its actual manufacturer was: Guangzhou Tengyu Cosmetics Co., Ltd.
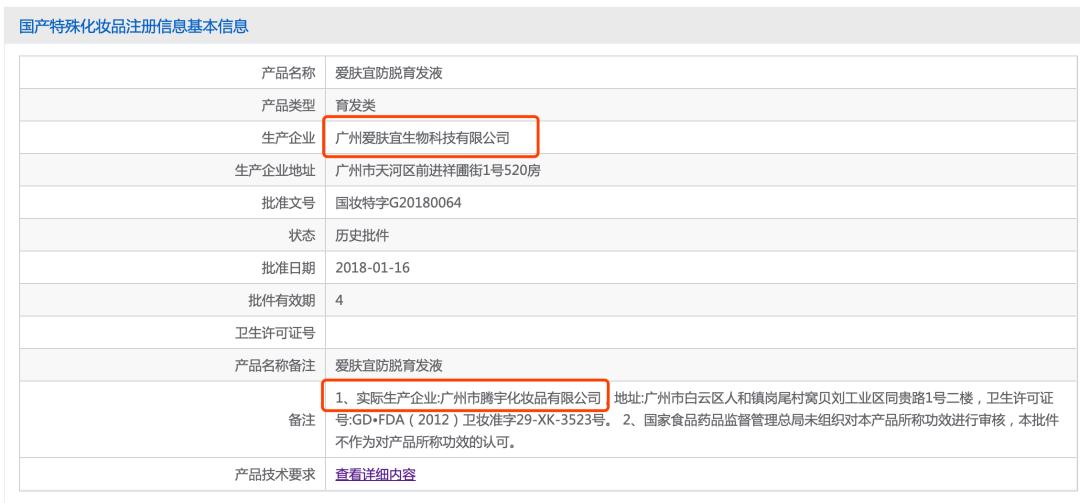
Enterprise investigation shows that Guangzhou Aifuyi and Guangzhou Tengyu have no equity relationship with Renhe Pharmaceutical. This is a typical OEM product.
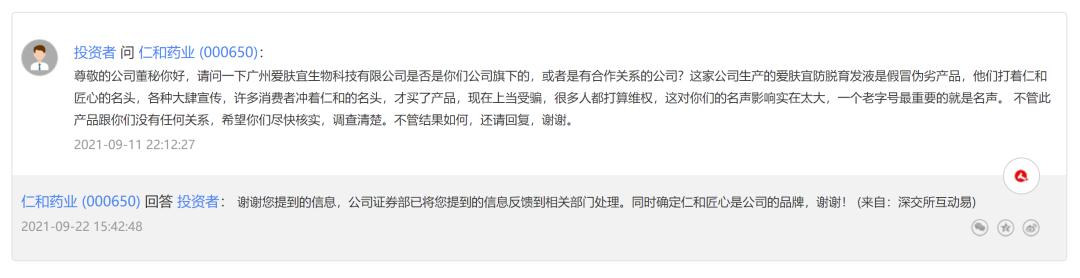
On the platform of investor interaction, many investors have raised a similar topic to Renhe Pharmaceutical, such as "What is the relationship between anti-alopecia lotion and Renhe Pharmaceutical", and they are concerned about the actual relationship between such branded products and Renhe Pharmaceutical.
02
The OEM model has spread to the medical field.
Today, the OEM model has been very popular in all walks of life. The most typical is the Antarctic people. Open a shopping platform, there will be many shops related to Antarctic people, and the products are countless, from shoes and clothing to bedding, from electric irons to gas stoves.
Antarctic people were originally a company that started from making underwear. Since 2008, Antarctic people have closed all the production lines of their own factories, and since then they have changed from "selling products" to "brand authorization", earning a lot of money. In 2019, Antarctic people earned 1.3 billion yuan through brand authorization. In 2020, the gross profit margin of Antarctic OEM service will reach 93.26%.
In recent years, the OEM model has spread to the medical and health care industries. Renhe, Tongrentang, Xiuzheng, Sunflower and many other well-known pharmaceutical companies increase their income through brand authorization.
Taking Renhe Pharmaceutical as an example, according to its 2020 annual report, the company achieved a total operating income of 4.106 billion yuan. The company’s products are divided into self-owned products and OEM products. From the perspective of income, self-owned products and OEM products basically account for half.
The OEM model supports half of the revenue of an old pharmaceutical company, which is enough to prove the dependence of well-known pharmaceutical companies on the OEM model.
Compared with R&D, OEM is obviously an easier shortcut. Accustomed to taking shortcuts, they are even more reluctant to take the difficult but correct path.
According to the third quarterly report of 2021 disclosed by Renhe Pharmaceutical, in the first three quarters, the company’s R&D expenses were 42.76 million yuan, and its revenue was about 3.659 billion yuan. The proportion of R&D expenses in revenue was only 1.1%. According to the annual report of Renhe Pharmaceutical for nearly 4 years, its annual sales expenses are 14-26 times as much as R&D expenses. Renhe’s characteristics of "emphasizing marketing and neglecting research and development" are often criticized by the outside world.
However, this is not the problem of Renhe. According to the survey report of the consulting industry in China, the R&D expenditure of large international pharmaceutical companies generally accounts for 15%-20% of the total sales, while the R&D investment of domestic pharmaceutical companies accounts for only 2%-3% of the sales revenue on average.
03
Want to win by branding? I’m afraid not.
Although the road to OEM is easy, it is not once and for all.
Just like Antarctic people, they thought they could "win" by brand authorization, but these two years have not been easy.
Since July last year, the share price of Antarctic e-commerce has dropped from the highest point of 24.41 yuan to around 7 yuan today, and its market value has evaporated by nearly 40 billion yuan. According to its 2021 semi-annual report, in the first half of 2021, the net profit of Antarctic e-commerce attributable to shareholders of listed companies was 246 million yuan, down 42.85% year-on-year.
The OEM model is like a double-edged sword, which not only contributes a lot of profit to the enterprise, but also brings various negative problems to the brand, such as product quality, word of mouth and even false propaganda.
There are many complaints about branded products of pharmaceutical companies on the websites of Black Cat Complaint and 315 Consumer Protection Complaint. Most of the complaints revolve around "invalid products", "fake goods", "no refund" and "false propaganda".
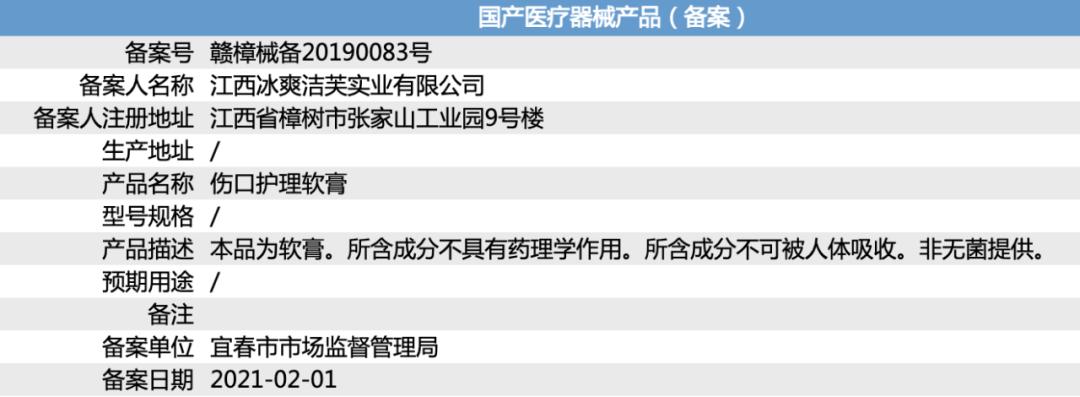
Minibus searched Pinduoduo for a wound care ointment. According to the industry details page, this product comes from a national brand-Amendment. Its function is "scar removal without leaving any trace", and it has been put on record by the Drug Administration, with good safety effect.
The actual manufacturer of this ointment is "Jiangxi Bingshuangjiefu Industrial Co., Ltd.", which has no equity relationship with Xiuzheng Pharmaceutical and belongs to OEM products.

According to the record number "Ganzhang Machinery Equipment No.20190083" provided by it, the minibus made an inquiry on the website of National Medical Products Administration, and the record information showed: "This product is ointment. The contained components have no pharmacological effects. The ingredients contained cannot be absorbed by the human body. Non-sterile. "
The "intended use" item is blank.
The minibus inquired about other brands of scar-removing ointment of the same type, and the column of "expected use" said "suitable for inhibiting and relieving hyperplastic scars caused by burns, trauma, surgery and other reasons".
In other words, this ointment probably has no scar removal effect. If so, then this product has the problem of false propaganda.
04
How can you post it? Who is responsible for the problem?
Who should be responsible for the quality problems of OEM products? The minibus consulted Chang Dongyue, the director of Shanghai Law and Craftsman Law Firm, and he thought that once the branded goods had quality problems, the brand supplier was the direct responsible person, and the processor was also liable under normal circumstances. Moreover, this is based on the quality problems of legally produced products, and it is necessary to bear civil liability.
However, more and more enterprises don’t talk about "martial arts", and many enterprises alienate OEM into pure lending brands, which is very problematic without any quality supervision on quality. In this case, if the illegal production leads to extremely bad social consequences, the brand and producer of OEM products not only need to bear civil liability, but also need to bear administrative responsibility and even criminal responsibility.
Chang Dongyue also said that the safety of food and drugs is related to people’s right to health and even life. Quality problems may cause irreversible damage to the body, and no matter how strict the quality supervision of food and drugs is. Regrettably, although China’s Food Safety Law, Drug Administration Law and Product Quality Law all stipulate product quality responsibility, the sanctions for violating food and drug safety are still too light, so that the food and drug field in China is still not safe enough. In this regard, the United States and Japan’s practice of using heavy codes to control food and drug safety is quite good and worth learning.

Relying on legal sanctions is the last step. As an enterprise, it should be its duty to abide by the rules. How can have no martial ethics, a post it?
According to Shi Lichen, the founder and strategic marketing expert of Beijing Dingchen Management Consulting Co., Ltd., OEM is not illegal, and the key is how to continue to control quality while authorizing. Whether you are OEM or OEM, consumers only know that they are buying your brand. Pharmaceutical companies should pay special attention to quality, otherwise, as the authorized products become more and more miscellaneous, the quality can not be controlled, and finally the reputation of the main business will be affected, which will not be worth the candle.
05
Difficulties and new ways for pharmaceutical companies
The advantages and disadvantages of OEM mode are very clear, and it can easily make money, but it is difficult to control the quality and the brand is easily damaged. The reason is very simple. The question is, those old pharmaceutical companies that have spent a hundred years settling down should have cherished their feathers. Why are they addicted to OEM?
Shi Lichen explained the reasons behind it: in recent years, the competition in the pharmaceutical industry has been fierce, and the operating pressure of pharmaceutical companies has increased. The old pharmaceutical companies have to find a way out. First, solve the fundamental problems and increase investment in research and development; Second, based on its own cost advantage, to achieve economies of scale. But these two roads are not easy to walk.
"In contrast, the OEM business has almost no cost and high net profit, so those pharmaceutical companies with brand awareness and strong premium ability naturally embarked on this road."
From this point of view, it is difficult for the old pharmaceutical companies to embark on the road of OEM, but this is not a long-term solution after all. Is it possible to open up a new road?
There is indeed a new road that has emerged-the field of cross-border beauty cosmetics and daily chemicals for pharmaceutical companies.
With the strong growth of functional skin care products, pharmaceutical companies such as Renhe, Pien Tze Huang, Tongrentang, Ma Yinglong, Yunnan Baiyao, Panlong Yunhai, Dong ‘e Ejiao and Xiuzheng Pharmaceutical have all set foot in the cosmetic and daily chemical industry.
Pien Tze Huang disclosed the 2020 financial report, and the company achieved operating income of 6.511 billion yuan, a year-on-year increase of 13.78%; The net profit was 1.672 billion yuan, a year-on-year increase of 21.62%. Behind the growth, the cosmetics sector contributed a bit. Its holding subsidiary Fujian Pien Tze Huang Cosmetics Co., Ltd. achieved revenue of 611 million yuan, up 42.01% year-on-year, and net profit of 114 million yuan, up 41.23% year-on-year.
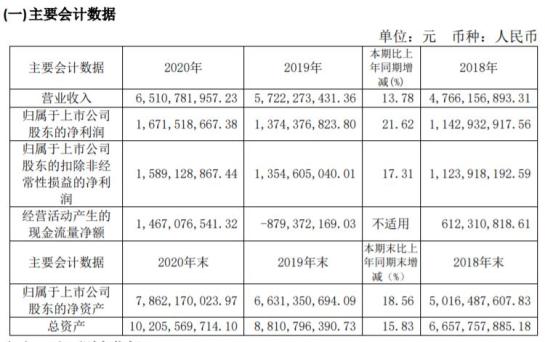
In July this year, Renhe Pharmaceutical announced that it planned to acquire 80% equity of seven cosmetics companies for 719 million yuan. One of them, Shenzhen Miura Natural Cosmetics, has been independently producing Renhe brand cosmetics, and some of the products distributed by the other six target companies come from this company.
In this regard, Shi Lichen analyzed that there is a huge Japanese industry called cosmeceuticals, which is not available in China, but we can make some functional cosmetics. Well-known pharmaceutical companies have natural advantages in making cosmetics across borders. For example, if brands can support the premium of products, they can get higher profit points. In addition, pharmaceutical companies themselves already have strong management teams, marketing teams and capital levels, which are not available to ordinary enterprises.
"The difference is nothing more than that some pharmaceutical companies, such as Renhe, have cultivated Renhe brand cosmetics related products through OEM before, and some products already have a certain market share, brand awareness and user base. At this time, it is logical to conduct mergers and acquisitions. Some pharmaceutical companies have had less involvement in this area before, so they may have to do it from scratch. "
In this way, pharmaceutical companies cross-border to make beauty cosmetics and daily chemical products. Originally, they just wanted to be a sideline through brand authorization. In the future, they may become one of their main businesses and gain new profit growth points by creating explosive IP products.
Author | Li Mengqing | Duty Editor | Zhang Wenlong
Editor | He Mengfei | Editor | Zheng Yuanmei | Tuyuan | vision china
Original title: "Old pharmaceutical companies learn from online celebrity brand. Have you ever bought such branded products? 》
Read the original text